Established, evidence-based clinical guidelines.
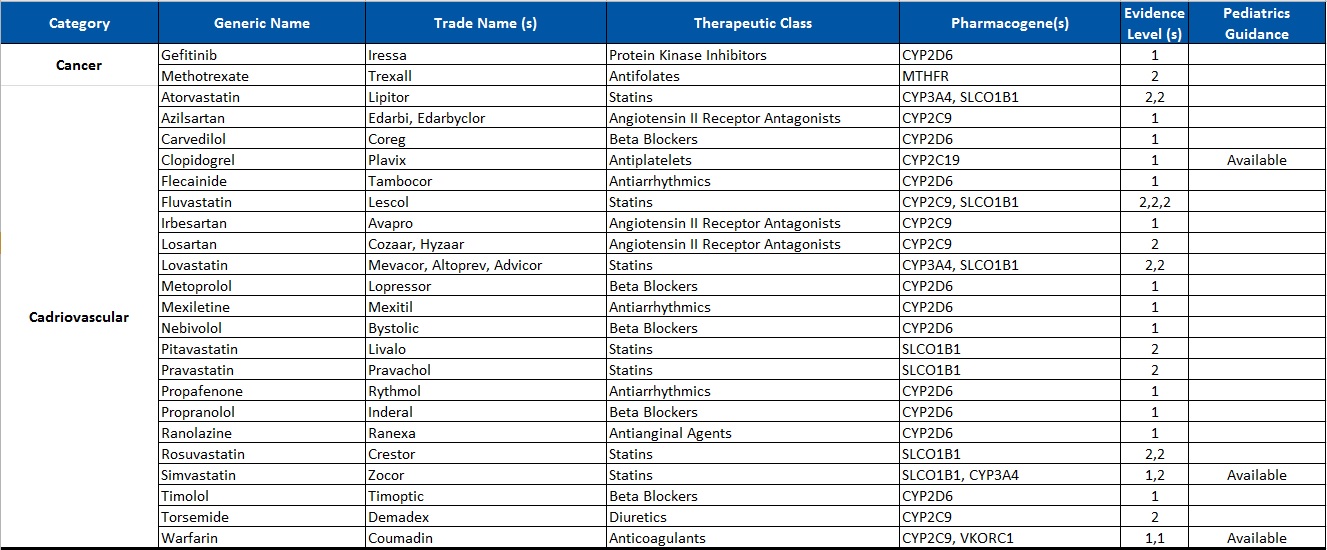
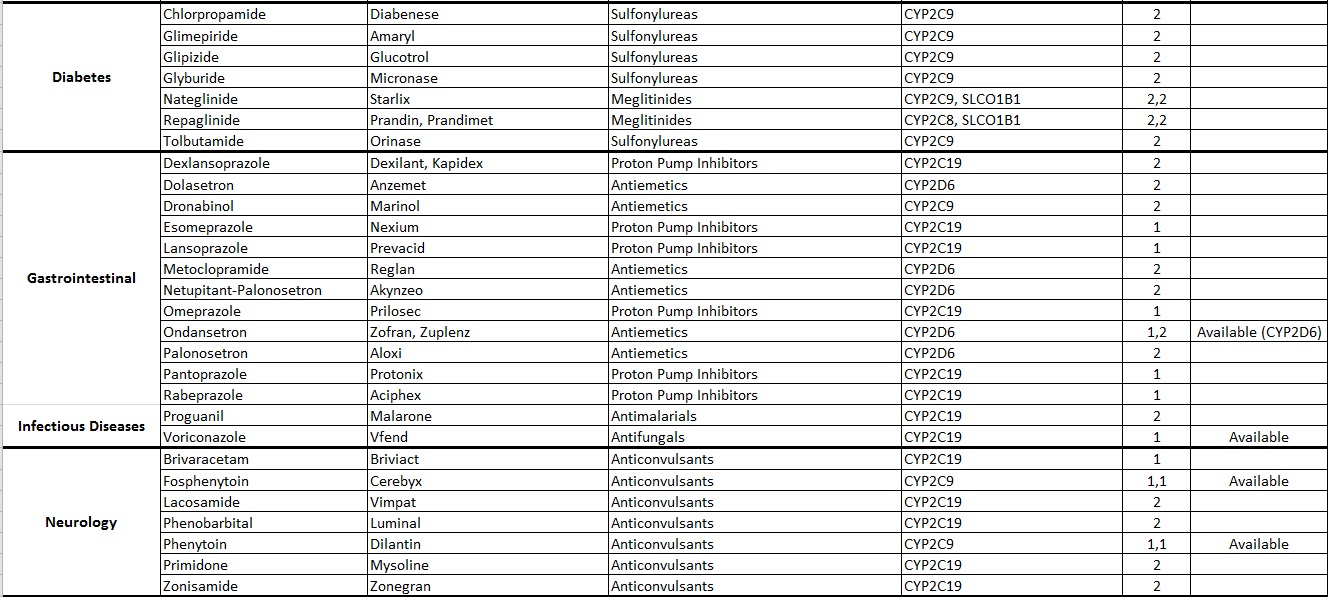
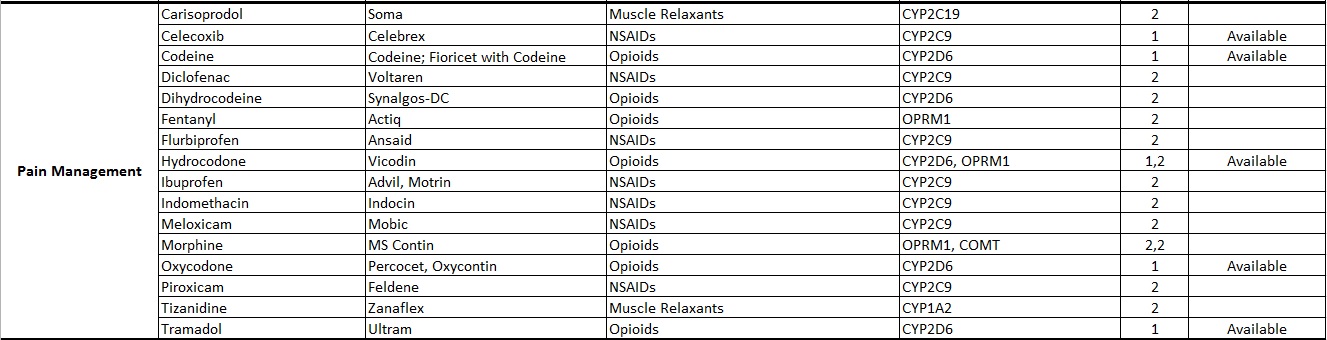
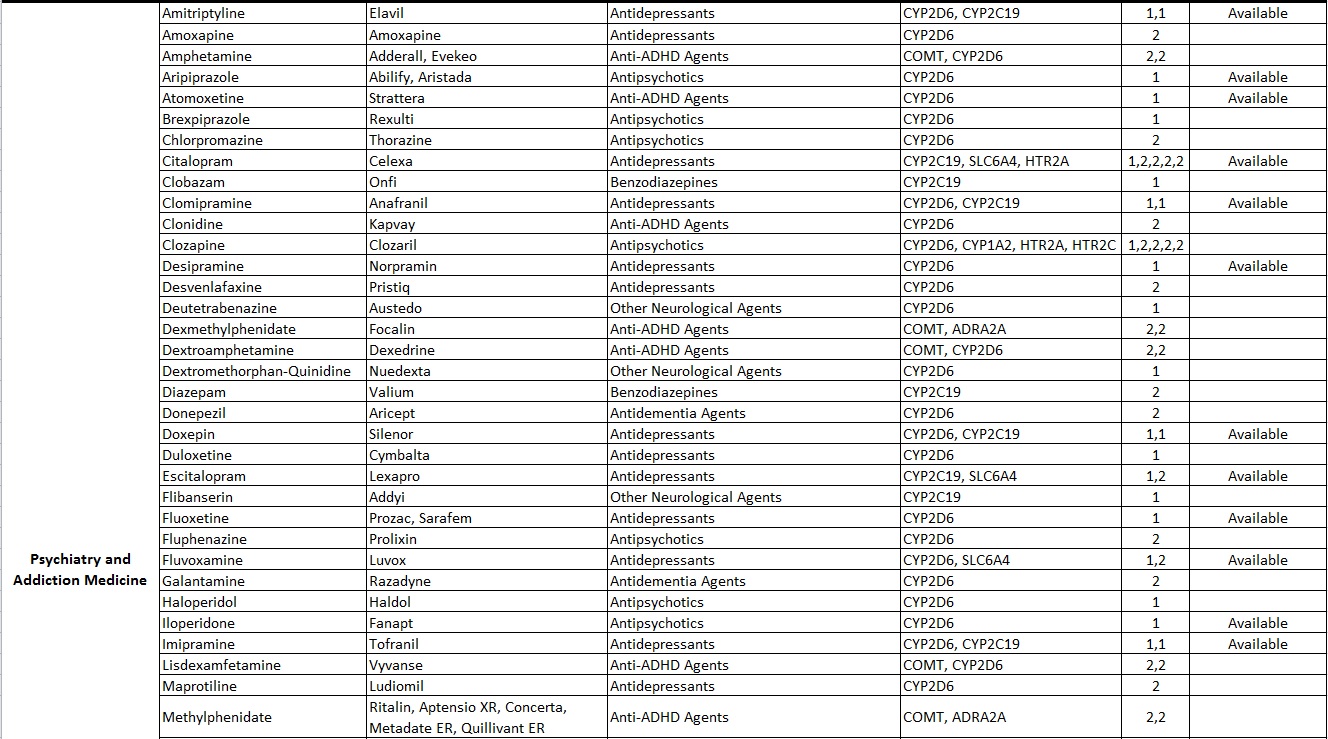
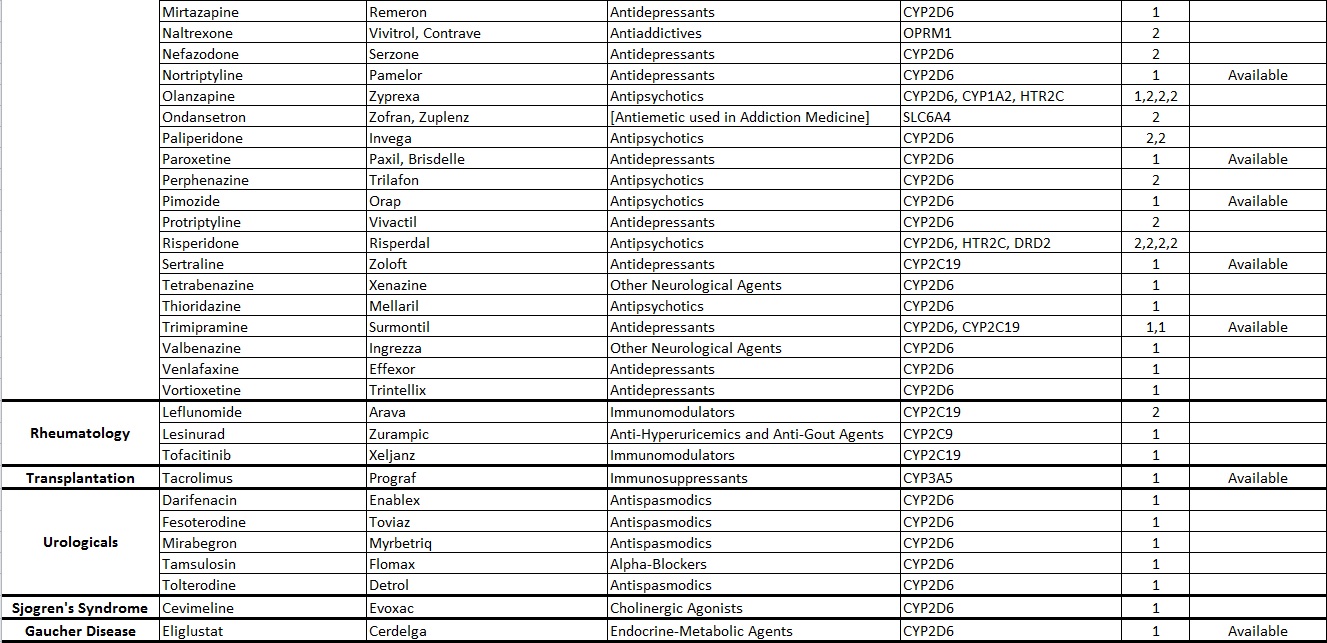
Common Drugs with Pharmacogenetic Associations reported at Alcala Labs:
Evidence Level 1:
Actionable
Recommendations extracted from evidence-based guidelines issued by international pharmacogenetic consortia, professional societies or regulatory bodies (CPIC, DPWG, FDA, EMA, CPNDS, ACMG)*. Recommendations suitable for implementation in a clinical setting. Guidelines may be updated as new knowledge arises.
* ACMG; American College of Medical Genetics and Genomics – CPIC; Clinical Pharmacogenetics Implementation Consortium- CPNDS; Canadian Pharmacogenomics Network for Drug Safety – DPWG; Dutch Pharmacogenetics Working Group- FDA; US Food and Drug Administration- EMA; European Medicines Agency
Evidence Level 2:
Informative
Drug-gene associations requiring further investigation.
There are no established, evidence-based guidelines issued by experts. The evidence documenting the genetic associations may be limited or insufficient. In some cases the evidence is still disputed. Recommendations are informative and implementation in a clinical setting is optional.