Now offering reliable COVID-19 and Respiratory Pathogens testing!
Click below to set up your on-site test collection appointment:
Alternatively, Alcala Labs call centers are ready to schedule your appointment.
Call us at (619) 450 5870 and speak to our Team at Alcala Labs.
(IMPORTANT: If you are ordering a test required for travel our team at Alcala Labs can provide you with guidance on which test to order. Please check with your airline and destination to ensure the correct test is ordered to match your travel needs. Current travel restrictions are diverse and can change daily. While Alcala Labs cannot be responsible for travel or booking cancellations based on test type or type result, the requirements for destination or airline bookings can be discussed to ensure the correct test is applied).
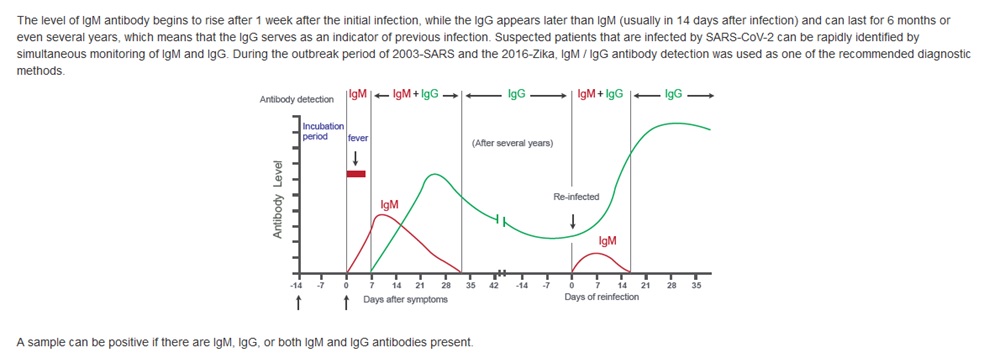
Alcala Labs is offering SARS-CoV-2 (COVID-19) testing to cover viral detection by COVID-19 RNA testing and antibody tests for IgG and IgM. Effectively covering the full spectrum of COVID-19 infectious disease progression is crucial in identifying affected individuals at different stages of a possible exposure.
Additionally, differential testing and determination of co-infections can be tested to rule out 20 other possible respiratory viral or bacterial pathogens.
CDC Coronavirus Self-Checker:
Utilize this interactive clinical assessment tool that will assist individuals ages 13 and older, and parents and caregivers of children ages 2 to 12 on deciding when to seek testing or medical care if they suspect they or someone they know has contracted COVID-19 or has come into close contact with someone who has COVID-19:
Clinical Test Information:
SalivaDirect™ is a real-time reverse transcription polymerase chain reaction (rRT-PCR) test intended for the qualitative detection of nucleic acid from SARSCoV-2 in saliva collected without preservatives in a sterile container from individuals suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories designated by the Yale School of Public Health, Department of Epidemiology of Microbial Diseases, that includes the Clinical Molecular Diagnostics Laboratory, Department of Pathology, Yale School of Medicine, located at 310 Cedar St., New Haven, CT 06510, that are also certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a and meet the requirements to perform high complexity tests. Alcala Labs is designated by the Yale School of Public Health to perform Saliva-Direct testing.
The panel is designed for specific detection of the 2019-nCoV N1 gene and the human RNase P gene (RP) as an internal control (two primer/probe sets total). RNA isolated and purified from saliva specimens is reverse transcribed to cDNA and subsequently amplified in the Applied Biosystems 7500 Fast Dx Real-Time PCR Instrument with SDS version 1.4 software.
Sensitivity: The Limit of Detection (LoD) study established the lowest SARS-CoV-2 viral concentration (Genomic Copy Equivalents or GCE = 3.00E+03 GCE/mL) that can be detected by the CDC Protocol at least 95% of the time using viral genomic RNA. 100% at 2X LoD.
Specificity: 100% PPV and NPV.
CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel – the oligonucleotide primers and probes for detection of 2019-nCoV were selected from regions of the virus nucleocapsid (N) gene. The panel is designed for specific detection of the 2019-nCoV (two primer/probe sets). An additional primer/probe set to detect the human RNase P gene (RP) in control samples and clinical specimens is also included in the panel. RNA isolated and purified from upper and lower respiratory specimens is reverse transcribed to cDNA and subsequently amplified in the Applied Biosystems 7500 Fast Dx Real-Time PCR Instrument with SDS version 1.4 software.
Sensitivity: The Limit of Detection (LoD) study established the lowest SARS-CoV-2 viral concentration (Genomic Copy Equivalents or GCE = 1.00E+03 GCE/mL) that can be detected by the CDC Protocol at least 95% of the time using viral genomic RNA. 100% at 3X LoD and 5X LoD.
Specificity: 100% PPV and NPV.
PHOENIXDX® COFLUENZA 4-PLEX IVD – is a real-time RT-PCR-based diagnostic test for the in vitro qualitative detection of Influenza A, Influenza B and SARS-CoV-2 in respiratory specimens and sera. Positive results indicate the presence of Influenza A, Influenza B or SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information must be considered to determine the actual patient infection status. Positive results do not exclude bacterial infection or co-infection with other viruses. Negative results do not exclude an infection with Influenza A, Influenza B or SARS-CoV-2 and must not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Sensitivity: LOD95 (Limit of Detection) defines the number of target sequences (copy number) that can be detected in ≥ 95% of reactions. The LOD95 was determined by testing a serial dilution of isolated SARS-CoV-2 RNA with 11 concentrations in 24 replicates per concentration. One copy of viral genomic RNA has been detected in 6 cases of 24 replicas. LOD95 for detection of SARS-CoV-2 is 2.75 copies/μL of the eluate. LOD95 for Influenza A (H3N2) is 20 copies/μL and LOD95 for Influenza B is 0.5 copies/μL of the eluate.
Specificity (tested against 60 different controls, e.g. viral, bacterial and human. including artificial SARS-CoV-2 genomic RNA and SARS CoV-2 isolates: SARS-CoV-2 = 100%, Influenza A = 96.63%, Influenza B = 98.48%.
FOSUN COVID-19 RT-PCR Detection kit – This EUA-approved method is a fluorescent probe-based Taqman RT-PCR assay system. The ORF1ab (Rdrp region included), N and E gene of SARS-CoV-2 will be detected qualitatively, including a separate internal reference. dUTP and UNG enzyme are used in the kit to prevent contamination of the amplified products. RNA isolated and purified from upper and lower respiratory specimens is reverse transcribed to cDNA and subsequently amplified in the Applied Biosystems 7500 Fast Dx Real-Time PCR Instrument with SDS version 1.4 software.
Sensitivity: was confirmed at 100% at 5.00E+03 GCE/mL and 100% Sensitivity at an LOD of at least 5.00E+03 GCE/mL (or 5000 NDU/mL = NAAT Detectable Units/mL).
Specificity: 100% PPV and NPV.
The NxTAG® CoV Extended Panel (NxTAG CoV) is an EUA-approved (Emergency Use Authorization) test authorized by the FDA for use by high-complexity, CLIA-certified laboratories such as Alcala Labs. This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. The NxTAG® CoV Extended Panel incorporates multiplex Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) with the Luminex® proprietary universal tag sorting system on the Luminex platform to easily detect SARS-CoV-2. Extracted total nucleic acid is added to pre-plated, Lyophilized Bead Reagents (LBRs) and mixed to resuspend the reaction reagents. The reaction is amplified via RT-PCR and the reaction product undergoes near simultaneous bead hybridization within the sealed reaction well. The hybridized, tagged beads are then sorted and read on the MAGPIX® instrument.
Sensitivity: The Limit of Detection (LoD) study established the lowest SARS-CoV-2 viral concentration (Genomic Copy Equivalents or GCE = 5.00E+03 GCE/mL) that can be detected by the NxTAG® CoV Extended Panel Assay at least 95% of the time using viral genomic RNA. 100% at 3X LoD and 5X LoD.
Specificity: 100% PPV and NPV.
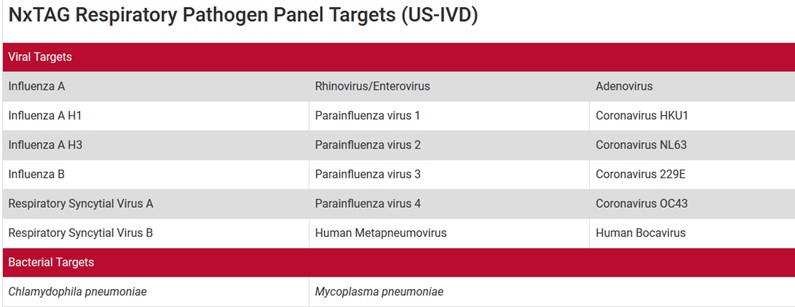
NxTAG® Respiratory Pathogen Panel (RPP) DNA/RNA swab test for In Vitro Diagnostic Use (IVD), incorporates multiplex Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) with the Luminex® proprietary universal tag sorting system on the Luminex platform to easily detect respiratory pathogen targets. Extracted total nucleic acid is added to pre-plated, Lyophilized Bead Reagents (LBRs), and mixed to resuspend the reaction reagents. The reaction is amplified via RT-PCR and the reaction product undergoes near simultaneous bead hybridization within the sealed reaction well. The hybridized, tagged beads are then sorted and read on the MAGPIX® instrument, and the signals are analyzed using the NxTAG Respiratory Pathogen Panel Assay File for SYNCT™ Software, providing a reliable, qualitative call for each of the 20 targets and internal controls within each reaction well.
COVID-19 IgG and IgM antibodies in venipuncture serum draws: are determined by COVID-19 ELISA Diagnostics kits developed by Epitope Diagnostics, Inc: KT-1032 EDI™ Novel Coronavirus COVID-19 IgG ELISA Kit and KT-1033 EDI™ Novel Coronavirus COVID-19 IgM.
The ELISA kits utilized for this test are designed, developed, and produced for the qualitative measurement of the human anti-COVID-19 IgG or IgM antibody in serum and utilizes the microplate based enzyme immunoassay technique. Assay controls and 1:100 or 1:10 diluted human serum samples are added to the microtiter wells of a microplate that was coated with COVID-19 recombinant full length nucleocapsid protein. After the first incubation period, the unbound protein matrix is removed with a subsequent washing step. A horseradish peroxidase (HRP) labeled polyclonal goat anti-human IgG tracer antibody is added to each well. After an incubation period, an immunocomplex of “COVID-19 recombinant antigen – human anti-COVID-19 IgG antibody – HRP labeled anti human IgG tracer antibody” is formed if there is specific coronavirus IgG antibody present in the tested specimen. The unbound tracer antibody is removed by the subsequent washing step. HRP tracer antibody bound to the well is then incubated with a substrate solution in a timed reaction and then measured in a spectrophotometric microplate reader. The enzymatic activity of the tracer antibody bound to the anti-COVID-19 IgG on the wall of the microtiter well is proportional to the amount of the anti-COVID-19 IgG antibody level in the tested specimen.
Epitope Diagnostics, Inc. confirms that the materials are compliant with the Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised) guideline issued on May 4, 2020. The products are still eligible for distribution as in vitro diagnostics to laboratories certified to perform high complexity testing, and at the point-of care when covered by the laboratory’s CLIA certificate for highcomplexity testing. The test kit method and performance criteria were submitted to the Federal Drug Administration (FDA) under two routes: Emergency Use Authorization (EUA) and NOTIFY per Section IV.D of the Policy for Diagnostic Tests for Coronavirus Disease-2019.
Test Performance IgG: Serum samples from two cohorts of patients were tested using the IgG ELISA kit at the Jiaxing City Center for Disease Control and Prevention and Zhejiang University Hospital. The combined cohort consisted of normal healthy patients with samples collected prior to the COVID-19 outbreak [December 3, 2019] (n = 54) and RT-PCR confirmed positive patients after the second week of the onset of the disease (n = 30). The results are as follows:
For IgG the diagnostic sensitivity is 100%, the diagnostic specificity is 100%, the negative predictive value is 100%, the positive predictive value is 100%.
Test Performance IgM: Serum samples from two cohorts of patients were tested using the IgM ELISA kit at the Jiaxing City Center for Disease Control and Prevention and Zhejiang University Hospital. The combined cohort consisted of normal healthy patients with samples collected prior to the COVID-19 outbreak [December 3, 2019] (n = 54) and RT-PCR confirmed positive patients after the second week of the onset of the disease (n = 20). The results are as follows:
For IgM the diagnostic sensitivity is 45%, the diagnostic specificity is 100%, the negative predictive value is 83.1%, the positive predictive value is 100%.
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is an EUA-approved lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood, plasma from anticoagulated blood (Li+ heparin, K2-EDTA and sodium citrate), or serum. The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) should not be used to diagnose acute SARS-CoV-2 infection. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C 263a, to perform moderate or high complexity tests.
Results are for the detection of SARS CoV-2 antibodies. IgM and IgG antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although the duration of time antibodies are present post infection is not well characterized. Individuals may have detectable virus present for several weeks following seroconversion. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
The sensitivity of COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) early after infection in unknown. Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary. False positive results for COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using a second, different IgG or IgM assay such as the Serum ELISA test described above.
The clinical performance of the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) was evaluated by testing a total of 191 plasma (K2-EDTA) clinical samples – 90 positive samples and 101 negative samples from individual patients exhibiting pneumonia, respiratory symptoms and fever etc. Testing was performed at two sites in China from January to mid-March 2020. COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) results for IgM and IgG detection were compared to the results of RT-PCR assays for SARSCoV-2 from oropharyngeal swabs (Site #1) and sputum (Site #2).
-
Measure
- IgM Sensitivity
- IgG Sensitivity
- (IgM+ or IgG+; Total) Sensitivity (PPA)
- (IgM-/IgG-; Total) Specificity (NPA)
- Cross-reactivity with HIV+
-
Estimate
- 100% (30/30)
- 96.7% (29/30)
- 100% (30/30)
- 97.5% (78/80)
- 0% (0/10) – not detected
-
Confidence Interval
- (88.7%; 100%)
- (83.3%; 99.4%)
- (88.7%; 100%)
- (91.3%; 99.3%)