Test Details: This two-step enzymatic reaction uses glucose oxidase, peroxidase and a chromogen. Glucose oxidase catalyzes the formation of gluconic acid and hydrogen peroxide via the oxidation of glucose. Peroxidase then catalyzes the reaction of hydrogen peroxide with a chromogen via the oxidation of chromogen to colors ranging between green to gray-blue. Other sugar compounds are not detected.
• Expected Values: Normal urine contains very little protein: usually less than 10 mg/dL is excreted. Positive protein results are considered pathological and should be investigated.
• Sensitivity: 20 mg/dL protein in urine
• Performance Characteristics: the test pad detects primarily albumin (also tested for in Blood specimens, see AlbP – Albumin). A negative result does not exclude the presence of other protein molecules such as Bence Jones proteins, globulin and mucoproteins (see TP – Total Protein test for Blood specimens).
• Limitations: Food dyes such as red beets and therapeutic pigments such as methylene blue and pyridium may mask the coloration of the test pad. Interference may occur with high specific gravity. Interference may also occur with disinfectants, wetting agents and blood substitutes (quartenary ammonium compounds, polyvinylpyrolidone, chlorohexidine). There is no interference from pH.
Test Details: This test is based on the “protein error” of pH indicators on the green color developed from the presence of protein. This dye-binding test is particularly strong with albumin.
Bilirubin
•
Expected Values: In normal urine, no detectable level of bilirubin should be obtained. Positive results require further investigation.
•
Sensitivity: 1.8 mg/dL bilirubin in urine (see
BiliT – Bilirubin (Total) or
Bilirubin (Direct) for tests performed in
Blood specimens)
•
Performance Characteristics: The test is specifically developed for bilirubin. Biliverdin does not react with this test pad.
•
Limitations: Some urine specimens may contain impurities such as food dyes and therapeutic pigments to produce a yellowish or reddish discoloration of the test pad that may lead to the interference. Elevated concentrations of nitrite may inhibit the reaction.
Bilirubin is light sensitive and prolonged exposure of urine specimens to light may result in diminished or false negative values. Elevated urobilinogen concentrations may slightly enhance the response of this test pad. Ascorbic acid concentrations ≥ 300 mg/dL may interfere with the test. MESNA concentrations ≥ 1140 mg/dL may cause false negative results.
Test Details: This test is based on the coupling of bilirubin with diazonium salt in an acidic medium. A pinkish tan color proportional to bilirubin concentration is generated.
Urobilinogen
• Expected Values: In normal urine, urobilinogen is usually present at concentration up to 1 mg/dL. A result of 2 mg/dL represents the transition from normal to abnormal state and the specimen should be investigated further for possible liver disease and hemolytic disorders.
• Sensitivity: 1.6 mg/dL urobilinogen in urine
• Performance Characteristics: The diazonium-based test is more specific for urobilinogen than Ehrlich’s reagent based- test. Test strips cannot determine the absence of urobilinogen, which may be significant in biliary obstruction.
• Limitations: This test is inhibited by elevated concentrations of formaldehyde and nitrite concentrations ≥ 10 mg/dL. Food dyes and medications that have an intrinsic red color in acidic medium such as red beets, azo dyes, phenazopyridine and p-aminobenzoic acid may produce false positive results. Prolonged exposure to light may lead to diminished or false negative values.
Test Details: This test is based on the coupling reaction of urobilinogen with a stable diazonium salt in buffer. A pink to red color proportional to the urobilinogen concentration is generated.
pH
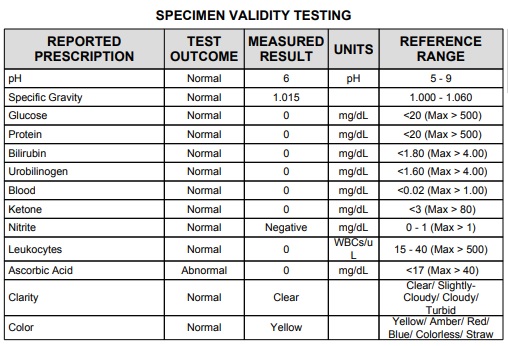
• Expected Values: The normal pH of urine can vary between pH 5.0 and pH 9.0
• Performance Characteristics: pH values are determined in units of 1.0 over the range from 5.0 to 9.0. A urine pH test can tell your doctor how acidic or basic (alkaline) your urine is using a simple, painless urine test. Many diseases, your diet, and the medicines you take can affect how acidic or basic your urine is. For instance, results that are either too high or low can indicate the likelihood that your body will form kidney stones. If your urine is at an extreme on either the low or high end of pH levels, you can adjust your diet to reduce the likelihood painful kidney stones will form. In short, your urine pH is an indicator of your overall health and gives your doctor important clues as to what is going on in your body.
Because certain medications can make your urine more acidic, your physician also may order the urine pH test to determine if the medicine is making your urine too acidic. The urine pH test can also be used to determine the best medication to prescribe when you have a urinary tract infection. For example, according to the National Institutes of Health, antibiotic medications such as streptomycin, neomycin, and kanamycin are most effective when your urine is alkaline.
• Limitations: Same as manual.
Test Details: This test contains a mixed indicator which assures a marked change in color between pH 5 and pH 9. Colors range from orange through yellow and green to cyan.
Blood
• Expected Values: Normal urine contains no detectable hemoglobin or intact red blood cells. Any positive results should be further evaluated.
• Sensitivity: 0.02 mg/dL blood in urine
• Performance Characteristics: Intact and lysed red blood cells are both detected by the assay, which contains lysing agents to lyse intact red blood cells releasing hemoglobin. A hemoglobin concentration of 0.015 – 0.062 mg/dL is approximately equivalent to 5 – 20 intact red blood cells per microliter (also see Hemoglobin (HGB) test provided for Blood specimens).
• Limitations: Reducing agents such as ascorbic acid, uric acid, glutathione and gentisic acid may cause false negative results. Samples with a pH of 5 may interfere with the test. Preservatives (formalin) and cleaning agents such as hypochlorite may result in false positives. High concentrations of nitrite can delay the reaction. There is no interference from specific gravity for this test. Ascorbic acid concentrations ≥ 300 mg/dL may interfere with the test. MESNA concentrations ≥ 1140 mg/dL may cause false negative results.
Test Details: This pseudo-enzymatic test contains organic peroxide and a chromogen. The peroxidase effect of hemoglobin and myoglobin causes a color change to green.
Ketone
• Expected Values: Detectable amounts of ketone do not appear in the urine of normal specimen. Positive ketone values may result from the following conditions: starvation, dietary imbalance, diabetes mellitus, eclampsia, insulin dosage monitoring, vomiting and other metabolic disorders.
• Sensitivity: 3 mg/dL ketone in urine
• Performance Characteristics: The test does not measure β-hydroxybutyric acid and is only slightly sensitive to acetone.
• Limitations: Elevated concentrations of phenylpyruvic acid may interfere with the test pad and produce a variety of colors. Phthaleins and anthraquinone derivatives exhibit a red color in alkaline medium and this may mask the response. Large amounts of levodopa and medications containing sulfhydryl groups may produce atypical color reactions. MESNA may produce false positive results.
Test Details: This test is based on Legal’s method in which the test pad contains sodium nitroprusside and glycine in an alkaline medium. A violet color proportional to methylketone is generated.
Nitrite
• Expected Values: Normal urine contains no detectable nitrite. However, a negative result does not rule out a urinary tract infection.
• Sensitivity: 0.04 mg/dL nitrite in urine
• Performance Characteristics: This test is specific for nitrite. Results may depend on the ability of bacteria to reduce nitrate to nitrite, the number of bacteria and the retention time of the urine in the bladder.
• Limitations: Food dyes and therapeutic pigments such as red beets and pyridium may cause false positive responses. A negative response in the presence of bacteriuria may be caused by the following: non-nitrite producing microorganisms, low nitrate diet, antibiotic therapy, strong diuresis, or insufficient urinary retention time in the bladder. There is no interference from pH or specific gravity for this test. Ascorbic acid concentrations ≥ 300 mg/dL may interfere with the test. MESNA concentrations ≥ 1140 mg/dL may cause false negative results.
Test Details: This test is based on modified Griess reaction in which nitrite in the urine reacts with amide to form a diazonium compound. The subsequent coupling reaction yields a pink color in the presence of nitrite. Some Gram positive and non-nitrite-forming bacteria are not detected in this test.
Leukocytes
• Expected Values: Normal urine specimen should not produce a positive result.
• Sensitivity: 15 WBCs/μL in urine (also see White Blood Cell count (WBC) test provided for Blood specimens).
• Performance Characteristics: Leukocyte test detects the presence of esterase in the granulocytic white blood cells. The test result is most frequently accompanied by the presence of bacteria that may or may not produce a nitrite positive reaction.
• Limitations: False positive results may occur in the presence of preservatives such as formaldehyde and formalin. High concentrations of protein, glucose, cephalexin and gentamicin may diminish the color response. Test results may be positive in the absence of observable cells if the granulocytes have lysed. The test can be negative in the presence of visible leukocytes if they have not lysed and/or are not granulocytes. There is no interference from specific gravity for this test. WBC readings at 75 cells/uL may be increased at pH 9. There is no interference from cephalosporins at 11 mg/dL. However, higher concentrations of cephalosporins may interfere with the test. Boric acid concentrations ≥ 500 mg/dL may interfere with the test. MESNA may produce false positive results.
Test Details: This enzymatic test pad contains an indoxyl ester and a diazonium salt. Granulocyte esterases react with indoxyl ester and diazonium salt to generate a violet color.
Specific Gravity
Your doctor will look at the ratio of the density of your urine to the density of water. To put it another way, the specific density of water itself would be 1.000. Ideally, urine specific gravity results will fall between 1.002 and 1.030 if your kidneys are functioning at a normal level
Specific gravity is physically determined by refractometry in the Urine Chemistry System and is not analyzed by strip chemistry.
Ascorbic Acid
• Expected values: Ascorbic acid is found in various food supplies and dietary supplements. Concentrations of ascorbic acid greater than 20 mg/dL can be expected to cause strong interference with glucose, blood and nitrite.
• Sensitivity: 17 mg/dL ascorbic acid in urine
• Performance Characteristics: The oxidized form, dehydroascorbic acid, does not react with this test pad.
• Limitations: Samples at a pH of 9.0 may interfere with the test. MESNA may produce false positive results.
Test Details: This test is based on Tillman’s reaction in which the presence of ascorbic acid leads to the decolorization of the test pad from gray-blue to orange.
Color and Clarity are measured directly from transmitted and scattered light in the sample using proprietary algorithms embedded in the Urine Chemistry System.
REFERENCES
1. Legal, E. A.: New Acetone Reaction and Its Applicability for the Examination of Urine. Chem. Centr. 14: 652 (1883)
2. Chertack, M. and Sherrick, J.: Evaluation of Nitroprusside Dip Test for Ketone Bodies. JAMA 167: 1621 (1958)
3. Roe, J. H.: Chemical Determination of Ascorbic, Dehydroascorbic and Diketogulonic Acids. Methods of Biochemical Analysis, Vol 1:115 (1954) ed. By D. Glick, Interscience Publishers, New York
4. Comer, J.: Semiquantitative Specific Test Paper for Glucose in Urine. Anal. Chem. 28:1748 (1956)
5. Appel, W., Nurck, C. and Merkle, U.: A Rapid Test for Urinary Glucose with an Ascorbic Acid Zone. Medical Laborartory 6: 29 (1979)
6. Sorenson, S.: The Measurement of Hydrogen Ion Concentration and Its Importance for Enzymatic Process. Biochem. Z. 21: 131 (1909)
7. Vonderschmitt, D. and Scholer, A.: Testreifen fur Screening-Untersuchungen zum semiquantitativen Nachweis von Proteinurien. J. Clin. Chem. Biochem. 19: 997 (1981)
8. Leonards, J.: Simple Test for Hematuria Compared with Established Test. JAMA 179: 807 (1982)
9. Weltmann, O.: Method for the Simple Detection of Urinary Tract Infections. Wien. Med. Wschr. 72: 618 (1922)
10. Strasinger, S. K. and De Lorenzo, M. S.: Urinalysis and Body Fluids. 4th edition, F. A. Davis Company, Philadelphia (2001)
11. Simerville, J.A., Maxted, W.C. and Pahira, J.J.: Urinalysis: A Comprehensive Review. A,. Fam. Physician 71: 1153 (2005)